A constellation of causes: on the multi-step theory of ALS
About ALS / On the multi-step theory
In search of the big picture
When determining ALS risk, genetic mutations may account for 1 piece of the puzzle.1,2 Steve Vucic, Professor of Medicine at the University of Sydney’s Westmead Clinical School, is committed to putting together the rest of the pieces.
“Genetic mutations are strong risk factors,” Prof. Vucic says. “They can predispose a person to a certain condition. But they are not, by themselves, causative. ALS does not develop as a result of mutations alone.” 2 Like many of his colleagues, Prof. Vucic subscribes to the theory of a complex, multi-step process in which genetic mutations play an important role, but in which several other factors are also at play. It is a kind of algorithm, with multiple variables, that helps to map how and why ALS may appear in some people with a genetic mutation, but not in others.1
“This multi-step hypothesis was adopted from a similar model developed in other disease states. They hypothesized that certain diseases are caused by multiple “hits” to the genomic material: if a patient gets a certain critical number of “hits”, the cell then mutates and causes the disease.”1-3
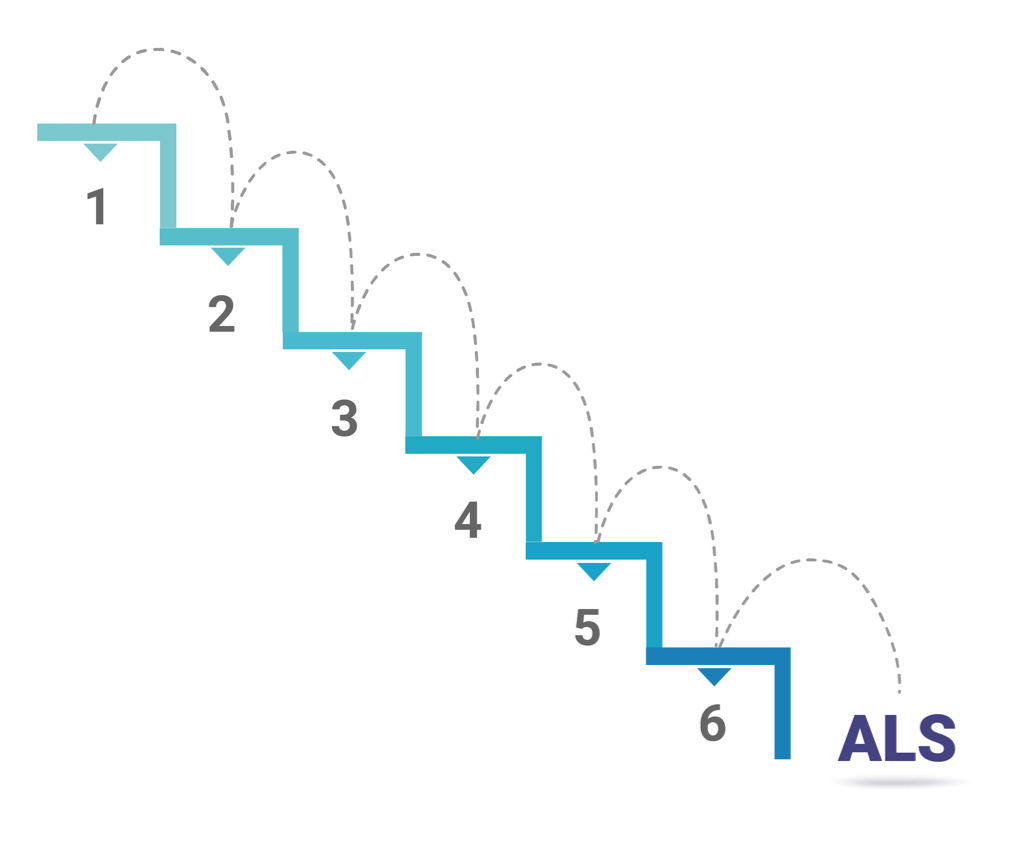
Prof. Vucic describes a process by which his friend and colleague performed a logarithmic analysis of data on ALS incidence and age of onset in Europe. His analysis revealed a linear and “remarkably reproducible” model for patients with ALS across different European cohorts. The model for these cohorts demonstrated that six steps—or “hits”—were required to produce a motor neuron disease like ALS.2
So, while the multi-step theory was already established in ALS, Prof. Vucic decided to apply it in his own research. “We wanted to see if we could reproduce this multi-step model with our own analysis of an Australian cohort,” he says. “We obtained 11 years of data from the Australian Institute of Health and Welfare and, remarkably, we reproduced those European findings. We saw once again that a person basically needed 6 steps to develop ALS.”4
Slope estimate expressing log incidence versus log age at onset
The initial analysis used age at death as a surrogate for age of onset. Prof. Vucic and his team hypothesized that it overestimated the number of steps required for onset, since death—by necessity—must come after onset. The researchers, therefore, re-analyzed the data by estimating the age of onset from a recently developed predictive model. As expected, the slope estimate was 5, indicating that 6 steps are required to trigger sporadic ALS. The 95% confidence intervals are highlighted in blue.
Adapted from Vucic S et al.4
After studying the data from the institute, Prof. Vucic and his team expanded their research to look at East Asian cohorts. Here, their mathematical modeling approach demonstrated a 5- and 6-step process in Japanese and South Korean patients, respectively. Prof. Vucic and his colleagues had now examined ALS patient data from three different continents—and all of it was saying basically the same thing.5
Counting the steps
“We now see that there are generally 6 sequential steps necessary to develop ALS, though that can vary,” says Prof. Vucic.
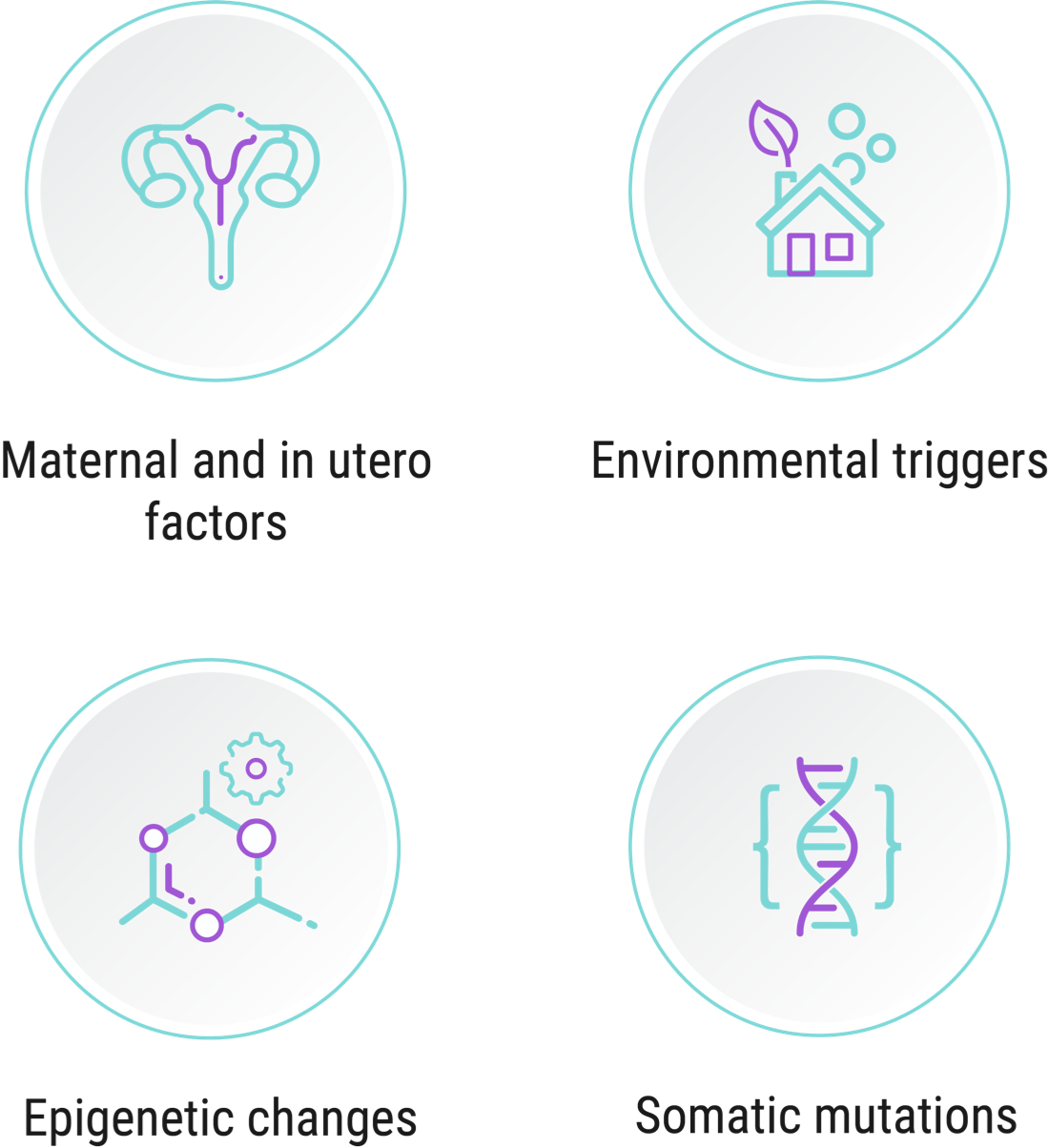
“We start with the genome. That’s step 1. Then there are maternal factors if those are involved, or in utero factors. That’s step 2. From there, patients' environments change. The activities they engage in change. Researchers believe that the next steps depend on when and if individuals are exposed to certain environmental factors or take part in certain physical activities that amount to ALS triggers.”1,4,6
The additional factors and activities Prof. Vucic alludes to that have been proposed to be associated with ALS may include cigarette smoking and military service.1 Any one of these factors could cause harm on its own, but for people already genetically predisposed to ALS, each could potentially constitute an additional step on the way to disease onset.1
Prof. Vucic cites the theory that the number of steps necessary to develop ALS can take place over the course of a life, from in utero, through childhood, and into adulthood, until the critical number of steps is reached and disease expression occurs.
“There are several epidemiological studies that have shown this sort of multiple factor model,” says Prof. Vucic. "The essence of the multi-step theory is that there isn’t one cause for motor neuron disease. There are multiple causes that may vary from person to person, and they play out at both a cellular and at a more macro level.”
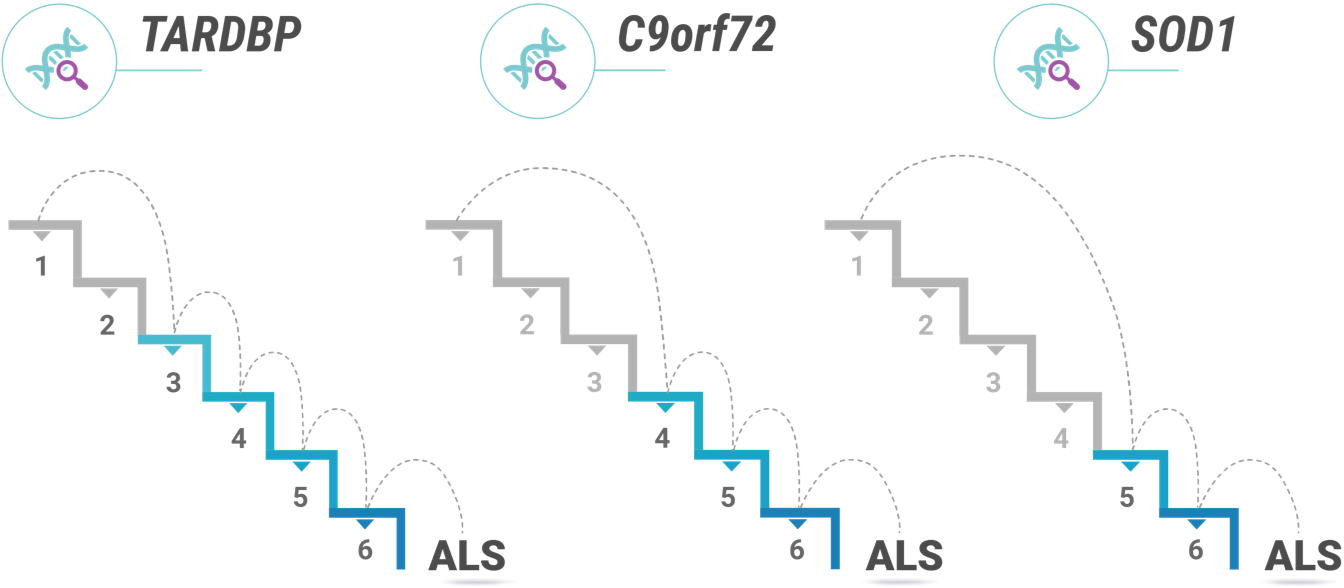
Prof. Vucic suggests visualizing the theory as a staircase, with a genetic ALS mutation as the first step. “It's a useful way to visualize it: this sort of climbing down and getting closer to disease onset with each step.”
Prof. Vucic both clarifies and caveats the stair metaphor by explaining that not all patients begin on the exact same step.
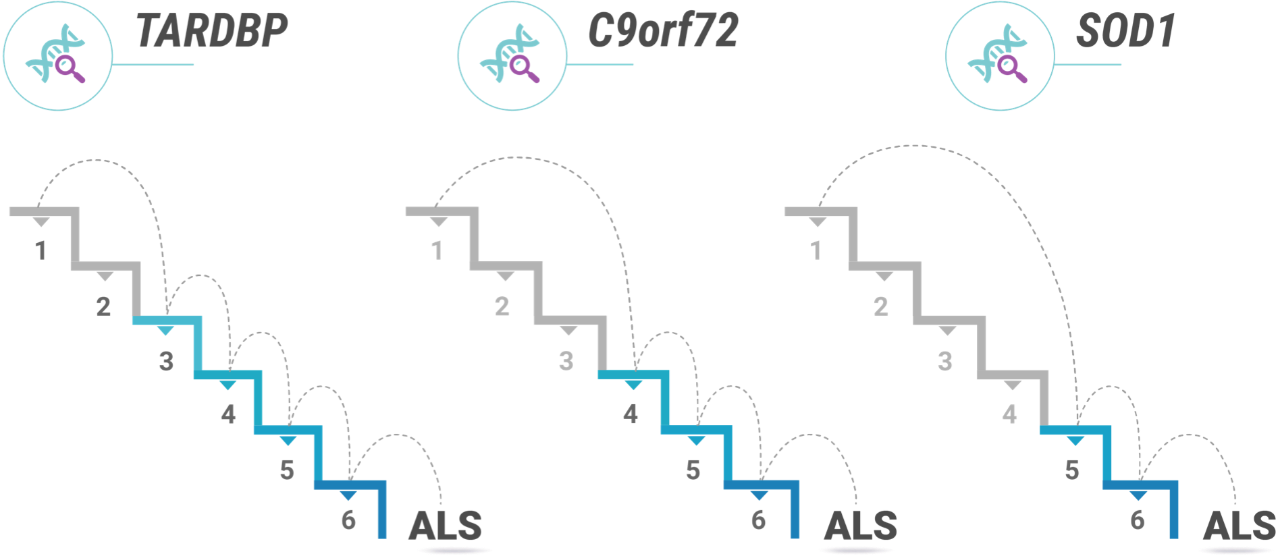
“Some patients with, say, a C9orf72 mutation may be starting at step four rather than step one. They might begin closer to disease onset.1 Instead of developing the disease in their 60s, one of these patients may develop it in their 40s. We see things like that happening.”
As an example, Prof. Vucic shares a finding from a cohort of ALS patients in Australia: females often needed one more step than males. This may, as Prof. Vucic argues, be related to differences in male and female hormones.4 Whatever the case may be, the finding from this cohort is in keeping with the fact that ALS is, in general, slightly more common in men than women.7 Prof. Vucic also touches on a study that showed patients in continental Africa with genetic ALS—including those with C9orf72, SOD1 and TDP-43 mutations—may have had fewer steps to develop the disease than the European, Australian, and Asian cohorts.8
Still, Prof. Vucic is wary of using the multi-step theory to make broad generalizations about individual patients or patient groups. “I don’t know how we would go about doing individualized disease steps. I don’t believe researchers have figured out how to look at a group of patients and say, ‘Well, you need four steps. You need five steps. You need seven steps.’ It is true that patients with the C9orf72 mutation sometimes have an earlier age of onset and more rapid disease progression.9,10 So in my mind, more malignant disease means fewer steps. On the other hand, there are certain SOD1 mutations that seem to come with a much better prognosis.11 This is all theoretical at this point, but these are the sorts of things researchers are working to understand.”
The multi-step process in practice
Prof. Vucic witnessed firsthand how the variable nature of the steps could play out through his experience with one family with the V149G genetic mutation.
“It’s a tragic story in which the grandmother had the onset of the disease in her 70s and died within a year,” Prof. Vucic explains. “Her daughter, the mother of our patients, experienced disease onset at the age of 50. And then a number of her children got the disease in their 20s and 30s and died within a year. You see that and you think, ‘What is going on here?’”
Witnessing the way ALS affected this family provided Prof. Vucic with a tangible and heartbreaking example of the role genetics may play in who develops ALS—and also of the degree to which it can’t explain everything. The fact that the disease manifested differently across generations indicates to him that there was a multi-step process at play.
“Historically we’ve observed a kind of pseudo-anticipation in the management of genetic ALS: this idea that subsequent generations will develop the disease at a similar age as the patients of a previous generation, and that the disease duration will be the same. But we saw in the case of this family that it doesn’t always work that way. Again, we see that genes can indeed be predisposing. But this is such a complex disease. Sometimes people ask, ‘What do you think causes ALS?’ I believe the multi-step theory teaches us to ask, ‘What do you think is the constellation of causes?’”
Prof. Vucic continues: “Genetics are one important aspect of how I believe we need to think about ALS. And so are the epigenetic and environmental factors associated with it. That's what I think this multi-step process tells us: we need an approach to clinical care in genetic ALS that is as multifaceted as the disease itself.”
References: 1. Chiò A, Mazzini L, D’Alfonso S, et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology. 2018;91(7):e635-e642. doi:10.1212/WNL.0000000000005996 2. Al-Chalabi A, Calvo A, Chio A, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 2014;13(11):1108-1113. 3. Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 2004;91(12):1983-1989. 4. Vucic S, Westeneng H-J, Al-Chalabi A, Van Den Berg LH, Talman P, Kiernan MC. Amyotrophic lateral sclerosis as a multi-step process: an Australia population study. Amyotrophic Lateral Scler Frontotemporal Degener. 2019;20(7-8):532-537. 5. Vucic S, Higashihara M, Sobue G, et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology. 2020;94(15):e1657-e1663. 6. Cooper D, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet. 2013;132(10):1077-1130. 7. Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162-172. 8. Luna J, Diagana M, Aissa LA, et al. Clinical features and prognosis of amyotrophic lateral sclerosis in Africa: the TROPALS study. J Neurol Neurosurg Psychiatry. 2019;90(1):20-29. 9. Esselin F, Mouzat K, Polge A, et al. Clinical phenotype and inheritance in patients with C9orf72 hexanucleotide repeat expansion: results from a large French cohort. Front. Neurosci. 2020;14:316. doi:10.3389/fnins.2020.00316 10. Miltenberger-Miltenyi G, Conceição VA, Gromicho M, et al. C9orf72 expansion is associated with accelerated decline of respiratory function and decreased survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90:118-120. doi:10.1136/jnnp-2018-318032 11. Parton M, Broom W, Andersen PM, et al. D90a-SOD1 mediated amyotrophic lateral sclerosis: a single founder for all cases with evidence for a Cis-acting disease modifier in the recessive haplotype. Human Mutat. 2002;20(6):473. doi:10.1002/humu.9081.PMID:12442272
1. Chiò A, Mazzini L, D’Alfonso S, et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology. 2018;91(7):e635-e642. doi:10.1212/WNL.0000000000005996 2. Al-Chalabi A, Calvo A, Chio A, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 2014;13(11):1108-1113. 3. Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 2004;91(12):1983-1989. 4. Vucic S, Westeneng H-J, Al-Chalabi A, Van Den Berg LH, Talman P, Kiernan MC. Amyotrophic lateral sclerosis as a multi-step process: an Australia population study. Amyotrophic Lateral Scler Frontotemporal Degener. 2019;20(7-8):532-537. 5. Vucic S, Higashihara M, Sobue G, et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology. 2020;94(15):e1657-e1663. 6. Cooper D, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet. 2013;132(10):1077-1130. 7. Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162-172. 8. Luna J, Diagana M, Aissa LA, et al. Clinical features and prognosis of amyotrophic lateral sclerosis in Africa: the TROPALS study. J Neurol Neurosurg Psychiatry. 2019;90(1):20-29. 9. Esselin F, Mouzat K, Polge A, et al. Clinical phenotype and inheritance in patients with C9orf72 hexanucleotide repeat expansion: results from a large French cohort. Front. Neurosci. 2020;14:316. doi:10.3389/fnins.2020.00316 10. Miltenberger-Miltenyi G, Conceição VA, Gromicho M, et al. C9orf72 expansion is associated with accelerated decline of respiratory function and decreased survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90:118-120. doi:10.1136/jnnp-2018-318032 11. Parton M, Broom W, Andersen PM, et al. D90a-SOD1 mediated amyotrophic lateral sclerosis: a single founder for all cases with evidence for a Cis-acting disease modifier in the recessive haplotype. Human Mutat. 2002;20(6):473. doi:10.1002/humu.9081.PMID:12442272