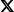Genetic penetrance and its implications for ALS
Genetic ALS / Genetic penetrance
Growing research on genetic ALS and the influence of environmental factors has advanced our understanding of the disease.1,2 Michael Elliott, MD, FAAN, Executive Medical Director of the Swedish Neuroscience Institute in Seattle, shares his outlook on genetic testing for patients with ALS and the implications of genetic penetrance when counseling patients.
Considerations for genetic testing
“One of the basics of this disease is that there is familial ALS, which constitutes about 10% of cases, and sporadic ALS—or disease that presents without a family history—which accounts for the other 90% of cases,”3 Dr. Elliott says. “In the past, I questioned the value of genetic testing. The thinking was, ‘If one does not have a family history of ALS, why bother to do genetic testing?’ But this sentiment has changed in recent years. Now I see more and more patients asking for genetic testing."
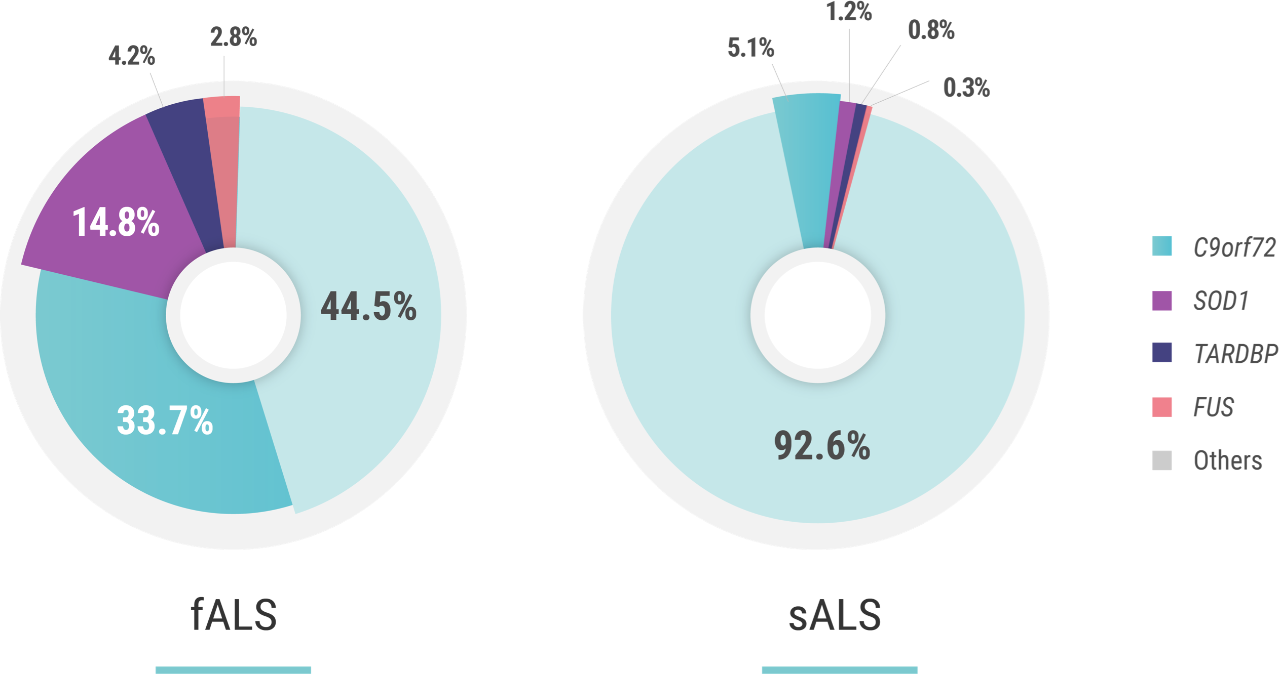
Clinical trials are currently recruiting genetic subpopulations of patients with ALS, including those with SOD1, FUS and C9orf72 expansion mutations.
“Of the 90% of cases that are considered sporadic, about 10% of those patients have a genetic component3,4 behind their ALS. And the point that I want to make is that this percentage may be growing.”4-6
The importance of penetrance
When asked about other important concepts to consider when identifying at-risk individuals through genetic testing, Dr. Elliott was quick to identify genetic penetrance. Penetrance is the proportion of individuals carrying a mutated gene that also express the affected phenotype.7
“I talk to patients about the idea of penetrance,” Dr. Elliott says. “For example, let’s say a patient has a mutation known to be associated with ALS. At age 50, they might show 50% penetrance, so you have about 50% chance at the age of 50 to manifest the disease and develop ALS. At 80, it is 100% penetrance. Some people never achieve 80 years so they would never show signs of the disease. Lower genetic penetrance is part of the reason why ALS sometimes doesn’t develop in patients who carry a mutation.”
The penetrance of C9orf72 hexanucleotide repeat expansion mutations—the most common of mutations associated with ALS—is estimated to be 60% at the age of 60 years and 91% at the age of 80 years. Penetrance can be attributed to multiple factors including the size of the GGGGCC expansion, DNA methylation, epigenetics, and the presence of additional mutations elsewhere in the genome.1,2
In a study of penetrance of the C9orf72 expansion based on a large review from published literature, data show that females may present with ALS symptoms later in life than males, suggesting that environmental, lifestyle, or hormonal factors may also affect penetrance patterns.7
Describing penetrance by age among C9orf72 carriers and identifying parameters that alter age of onset are important factors in better understanding this locus and to enhancing predictive counseling.7
The penetrance of SOD1 mutations—the second most-common group of mutations associated with ALS—is also variable. For example, in a study on pedigrees dating back to the 18th century, carriers of p.E101G, p.I114T, and p.V149G SOD1 mutations were reported to have a penetrance of >95% at the age of 78 years. Similarly, the penetrance of the p.A5V mutation, the most common SOD1 mutation in the US, has been estimated to be 91% at the age of 80 years. Other mutations have a reduced penetrance; an example is the p.D91A SOD1 mutation.2
All of these insights about genetic penetrance place an increased emphasis on genetic testing as a possibility for patients with ALS, and may have implications when discussing patient prognosis.
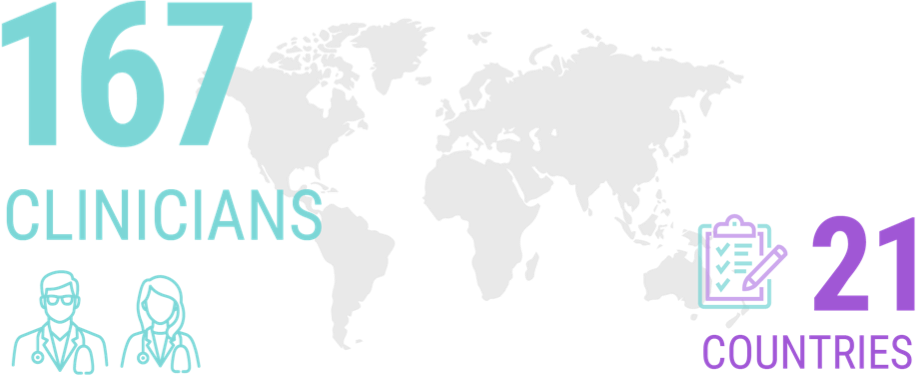
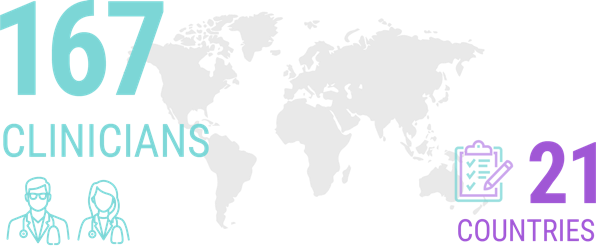
A survey of 167 clinicians from 21 different countries (the majority of whom identified themselves as having a special interest in ALS) revealed that more than half of them would seek genetic testing for themselves if they received an ALS diagnosis.8
Through genetic testing, it may now be possible for patients with genetic ALS to better understand the genetic component of their disease, and to share this knowledge with relatives to allow them to make decisions about learning their own genetic status. It can bring a degree of understanding and accommodation to what is otherwise a random and unexplained diagnosis—and inform participation in valuable clinical trials.6,9
References: 1. Roggenbuck J, Quick A, Kolb SJ. Genetic testing and genetic counseling for amyotrophic lateral sclerosis: an update for clinicians. Genet Med. 2017;19(3):267-274. 2. Chiò A, Mazzini L, D’Alfonso S, et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology. 2018;91(7):e635-e642. doi:10.1212/WNL.0000000000005996. 3. Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6:171. 4. Volk E, Weishaupt JH, Andersen PM, Ludolph AC, Kubisch C. Current knowledge and recent insights into the genetic basis of amyotrophic lateral sclerosis. Med Genet. 2018;30:252-258. 5. Boylan K. Familial amyotrophic lateral sclerosis. Neurol Clin. 2015;33(4):807-830. 6. Shepheard SR, Parker MD, Cooper-Knock J, et al; on behalf of Project MINE Consortium; Project MinE. Value of systematic genetic screening of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2021;jnnp-2020-325014. doi:10.1136/jnnp-2020-325014. 7. Murphy NA, Arthur KC, Tienari PJ, Houlden H, Chiò A, Trainor BJ. Age-related penetrance of the C9orf72 repeat expansion. Sci Rep. 2017;7(1):2116. doi:10.1038/s41598-017-02364-1. 8. Vajda A, McLaughlin RL, Heverin M, et al. Genetic testing in ALS: a survey of current practices. Neurology. 2017;88(10):991-999. 9. Benatar M, Stanislaw C, Reyes E, et al. Presymptomatic ALS genetic counseling and testing: experience and recommendations. Neurology. 2016;86(24):2295-2302.
1. Roggenbuck J, Quick A, Kolb SJ. Genetic testing and genetic counseling for amyotrophic lateral sclerosis: an update for clinicians. Genet Med. 2017;19(3):267-274. 2. Chiò A, Mazzini L, D’Alfonso S, et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology. 2018;91(7):e635-e642. doi:10.1212/WNL.0000000000005996. 3. Zarei S, Carr K, Reiley L, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015;6:171. 4. Volk E, Weishaupt JH, Andersen PM, Ludolph AC, Kubisch C. Current knowledge and recent insights into the genetic basis of amyotrophic lateral sclerosis. Med Genet. 2018;30:252-258. 5. Boylan K. Familial amyotrophic lateral sclerosis. Neurol Clin. 2015;33(4):807-830. 6. Shepheard SR, Parker MD, Cooper-Knock J, et al; on behalf of Project MINE Consortium; Project MinE. Value of systematic genetic screening of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2021;jnnp-2020-325014. doi:10.1136/jnnp-2020-325014. 7. Murphy NA, Arthur KC, Tienari PJ, Houlden H, Chiò A, Trainor BJ. Age-related penetrance of the C9orf72 repeat expansion. Sci Rep. 2017;7(1):2116. doi:10.1038/s41598-017-02364-1. 8. Vajda A, McLaughlin RL, Heverin M, et al. Genetic testing in ALS: a survey of current practices. Neurology. 2017;88(10):991-999. 9. Benatar M, Stanislaw C, Reyes E, et al. Presymptomatic ALS genetic counseling and testing: experience and recommendations. Neurology. 2016;86(24):2295-2302.